Write the Ions Present in Solution of Agno3
Now the moles of chloride ions needed to precipitate the silver ions is 283 10³ mol Ag 1 mol Cl1 mol Ag 2825 10³mol Cl. In Na_2CO_3 we get 2 sodium ions and 1 carbonate ion CO_32-.
Solved 004 Fa Vers Cat Part B Ion Of Nh Write The Ions Chegg Com
In MgSO_4 we get a magnesium ion Mg2 and a sulphate ion SO_42-.
. Write the ions present in solution of AgNO3. A positive Sol of AgI will form Ag will be preferred. If ceAgNO3 solution is added to an acidified unknown solution a white precipitate indicates the presence of ceCl ions a cream-colored precipitate indicates the presence of ceBr ions and a yellow precipitate indicates the presence of ceI ions Figure PageIndex1.
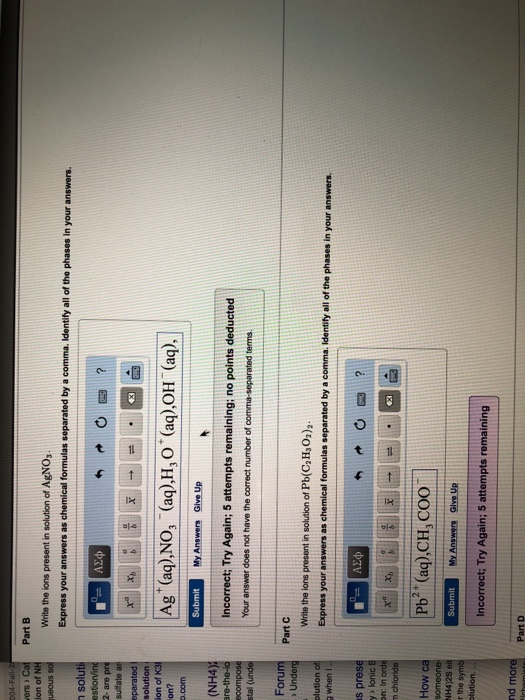
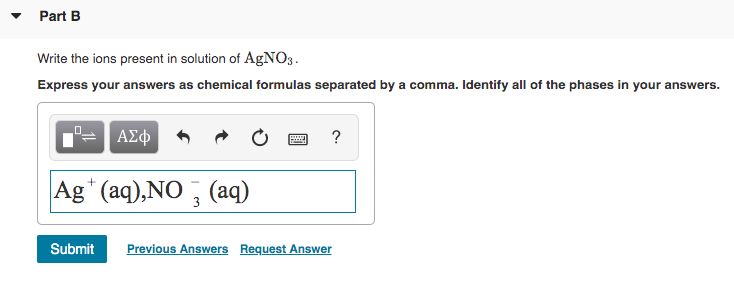
In NaCl we get the sodium ion Na and the chloride ion Cl-. AgNO3 NaCl - AgCl NaNO3 all one to one get moles AgNO3 382 moles NaCl 1 mole AgNO31 mole NaCl 382 moles AgNO3 ------------------------------- Molarity moles. Part B Write the ions present in solution of AgNO3.
_____ You will then dry and weigh the silver chloride. Separate aqueous ionic compounds into their constituent ions. Express your answers as chemical formulas separated by a comma.
Ions in a solution react with each other to form a new substance. Write the ions present in the solution of AgNO3. The PbCl2 s remains as one unit.
Use the above equation and Fe3aq SCN-aq FeSCN2aq to explain the observations in terms ofLeChâteliers Principle3 pts Observation after adding NaOH. In NH_4Cl we get an ammonium ion NH_4 and a chloride ion Cl-. Solve any question of Surface Chemistry with-.
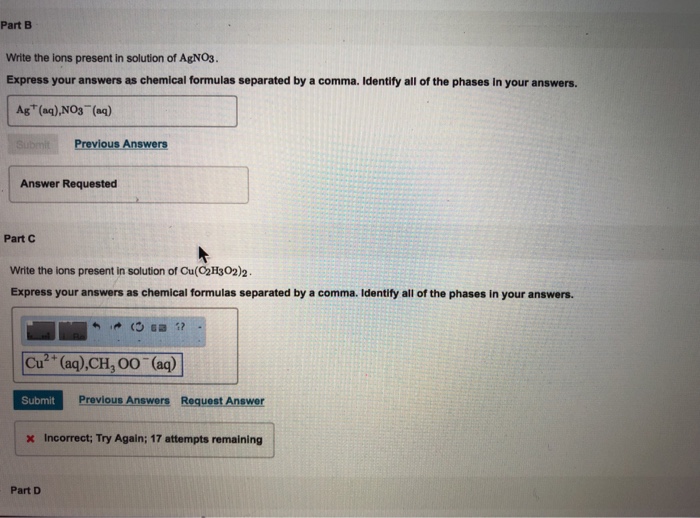
As we now know this reaction is called a precipitation reaction. BWrite the ions present in solution of CuC2H3O22. Express your answers as chemical formulas separated by a comma.
The solute is broken down completely into individual ions or molecules. Identify all of t LIMITED TIME OFFER. PbNO32 aq 2LiCL aq ---- PbCl2 s 2LiNO3 aq Write the complete ionic equation and a net ionic equation for the reaction.
Identify all of the phases in your answers. Cu2aq C2H3O2-aq 170 mL of a 030 M copper II acetate solution is mixed with an excess of a sodium sulfide solution How many grams of copper II. Express your answers as chemical formulas separated by a comma.
Up to 256 cash back Get the detailed answer. Identify all of the phases in your answers. In water AgNO3 disassociates into Ag ions and NO3- ions.
GET 20 OFF GRADE YEARLY. There are no spectator ions in this reaction. Based on the given chemical formula the ionic component of the compound are Pb² and 2 ions of NO₃².
What type of reaction is agno3 HCl. Express your answers as chemical formulas separated by a comma. Balanced equation first.
Silver nitrate or AgNO3 mixed with distilled water is a solution. A Write the ions present in solution of AgNO3. Using the ions present in the solution write a net ionic equation that would explain the precipitate observed.
3 pts Observation after adding AgNO3. Identify all of the phases in your answers. AgNO3 aq HCl aq AgCl s HNO3 aq Net ionic equation.
Click hereto get an answer to your question Write the cations and anions presentif any in the following compoundsa CH3COONa b NaCl c H2 d NH4NO3. Answer 1 of 2. Part B Write the ions present in solution of AgNO3- Express your answers as chemical formulas separated by a comma.
The decomposition of the given compound Pb NO32 will yield 3 different simpler compounds which are lead oxide PbO nitrogen dioxide NO2 and oxygen O2. LiClaq AgNO3aq AgCls LiNO3aq A. Identify the spectator ions in the following molecular equation.
Consider this precipitation reaction occurring in aqueous solution. But they do react. Ag aqNO3 aq Previous Answers Answer Requested Part C Write the ions present in.
This happens due to adsorption of Ag ions from the dispersion medium on the precipitate of silver iodide. Express your answers as chemical formulas separated by a comma. Other questions on the subject.
In each case these oppositely charged ions are held together by strong ionic bonds and the. D Write the ions present in solution of NH4Cl. Correct option is D When KI is added to AgNo 3.
Chemical compounds that have a charge are called ions. Express your answer as a chemical formula. After the silver has dissolved you will add hydrochloric acid solution to precipitate silver ions as silver chloride.
- AEC O O Ag aqNO. This is similar to salt or sugar being soluble in water. Of moles of silver ions found in silver nitrate solution is 250 10² mL 00113 mol Ag1000 ml solution 283 10³mol Ag.
Up to 256 cash back Get the detailed answer. Write the ions present in the solution of AgNO 3. Identify all of the phases in your answers.
Identify all of the phases in your answers. Aq Submit Previous Answers Request Answer Part Write the ions present in solution of PbC2H3O22. Write the chemical formula for the cation present in the aqueous solution of AgNO3.
If we write the reaction Na_2CrO_4 2AgNO_3 - Ag_2CrO_4 2NaNO_3 From the data given lets calculate moles ofAgNO_3 No. Express your answers as chemical formulas separated by a comma. C Write the ions present in solution of KNO3.
Write the ions present in the solution of AgNO3. Write the ions present in solution of agno3. List the two type of transporst that the cell in orde to transport molecules acroos the.
If moles fracMV 1000 frac01751000 00075 moles From the reaction it is clear that 2 moles of AgNO_3 is precipitated by. Chemistry 21062019 1930 jalenshayewilliams. Express your answers as chemical formulas separated by a comma.

Solved Part B Write The Ions Present In Solution Of Agno3 Chegg Com
Solved Part B Write The Ions Present In Solution Of Agno3 Chegg Com

Spectator Ions In Aqueous Solution Chemical Equation Solutions Physical Chemistry

Comments
Post a Comment